Predictors of weight loss and weight regain after bariatric surgery
Prediktory poklesu a opätovného nárastu hmotnosti po bariatrickom chirurgickom zákroku
Bariatrická chirurgia je u obéznych pacientov účinnou metódou na dosahovanie udržateľného poklesu hmotnosti, ako aj zlepšenie komorbidít súvisiacich s obezitou. Opätovný nárast hmotnosti sa môže vyskytnúť u významného počtu pacientov po bariatrickom/chirurgickom zákroku. K závažnej recidíve môžu prispieť nedodržiavanie diétnych opatrení, poruchy duševného zdravia, fyzická inaktivita, hormonálne/metabolické abnormality a problémy súvisiace s chirurgickým zákrokom. V súčasnej literatúre nájdeme množstvo klinických štúdií, ktoré skúmali prediktory poklesu a opätovného nárastu hmotnosti po bariatrickej operácii. Poruchy príjmu potravy a strata kontroly nad jedlom patria medzi príčiny, ktoré vedú k nižšiemu úbytku hmotnosti a väčšiemu nárastu hmotnosti. V štúdii SOS (Swedish Obese Subjects) boli u osôb po bariatrických/chirurgických zákrokoch v súvislosti s úbytkom hmotnosti a opätovným nárastom hmotnosti testované sekvenčné variácie DNA v jedenástich kandidátnych génoch obezity. Analýzy ukázali, že jenodnukleotidový polymorfizmus (single nucleotid polymorphism – SNP) v FTO géne súvisel s maximálnou redukciou hmotnosti po gastrickej bandáži. Iné štúdie potvrdili, že prediktormi opätovného nárastu hmotnosti po Roux-en-Y gastrickom bypasse boli nočné jedenie, depresia, vek a počiatočný BMI (index telesnej hmotnosti), typ chirurgického zákroku, čas od operácie, priemer stómie, úroveň nutričného poradenstva a fyzická inaktivita. Systematický prehľad štúdií, ktoré hodnotili zmeny koncentrácií leptínu, ghrelínu a inzulínovej senzitivity počas redukcie hmotnosti, testovali aj vzťah medzi týmito zmenami a opätovným nárastom hmotnosti. Autori systematického prehľadu dospeli k záveru, že samotné zmeny nestačia na predikciu opätovného nárastu hmotnosti po jej predchádzajúcej redukcii. Bez ohľadu na prediktory pre redukciu hmotnosti, udržanie redukovanej hmotnosti a opätovný nárast hmotnosti, esenciálnou podmienkou ostáva adherencia pacienta k liečbe s nadväzujúcim programom.
Kľúčové slová:
chirurgia obezity, nárast hmotnosti, obezita, pokles hmotnosti, prediktory
Doručené do redakcie: 2. 3. 2018
Prijaté po recenzii: 5. 4. 2018
Authors:
Volkan Demirhan Yumuk
Authors‘ workplace:
Istanbul University – Department of Internal Medicine (Cerrahpasa Faculty of Medicine), Division of Endocrinology, Metabolism and Diabetes, Turkey
Published in:
Forum Diab 2018; 7(2): 122-125
Category:
Review Article
Overview
Bariatric surgery in obese patients is effective in producing sustainable weight loss and improvement in obesity-related co-morbidities. However, weight regain can be seen in a substantial number of patients following bariatric surgery. Dietary non-compliance, mental health disorders, physical inactivity, hormonal/metabolic abnormalities and surgery related issues are among the underlying etiologies contributing to weight recidivism. Current literature is comprised of numerous clinical trials studying predictors of weight outcomes after bariatric surgery. Binge eating disorder and loss of control of eating are among those that result in less weight loss and more weight regain. DNA sequence variations in eleven obesity candidate genes were tested to display the association with weight loss and weight regain in the Swedish Obese Subjects bariatric surgery cases and the analyses revealed that the single nucleotide polymorphism in the Fat mass and Obesity associated gene was associated with maximum weight loss after banding surgery. In other studies night eating, depression, age and initial body mass index of the patient, surgical procedure, time since surgery, stoma diameter, nutritional counseling level and physical inactivity were shown to be the predictors of weight regain after Roux-en-Y gastric by-pass. A systemic review of studies assessing changes in leptin, ghrelin and insulin sensitivity during weight loss and testing the relationship between such changes and weight regain has concluded that these changes taken alone are not sufficient to predict weight regain following weight loss. Whatever predictors have been proposed so far for weight loss, maintenance and weight regain, patients’ adherence to treatment and follow up programs remains to be essential.
Key words:
obesity, obesity surgery, predictors, weight gain, weight loss
Introduction
The use of surgical weight-loss procedures has escalated since high morbidity and mortality rates are associated with a Body Mass Index (BMI) greater then and equal to 40 kg/m2 and with a BMI of 35 to 39 kg/m2 in the presence of a coexisting condition. Although more effective than lifestyle and pharmacologic interventions these procedures are associated with greater risks. Three main types of bariatric surgery (BS) are currently performed worlwide: laparoscopic gastric banding, sleeve gastrectomy and gastric by-pass [1]. Bariatric surgery in obese patients is effective in producing sustainable weight loss and improvement in obesity-related co-morbidities. However, weight regain can be seen in a substantial number of patients following bariatric surgery. Dietary non-compliance, mental health disorders, physical inactivity, hormonal/metabolic abnormalities and surgery related issues are among the underlying etiologies contributing to weight recidivism (tab. 1) [2]. Current literature is comprised of numerous clinical trials studying predictors of weight outcomes after bariatric surgery.
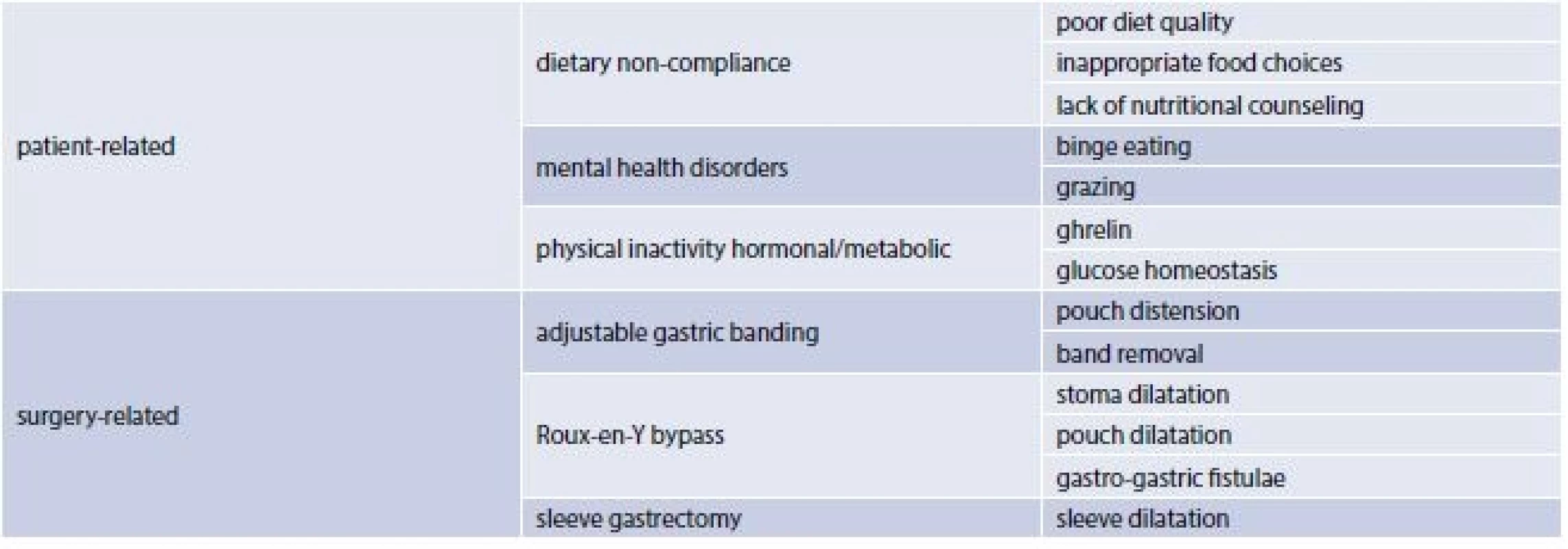
Predictors of weight outcomes after bariatric surgery
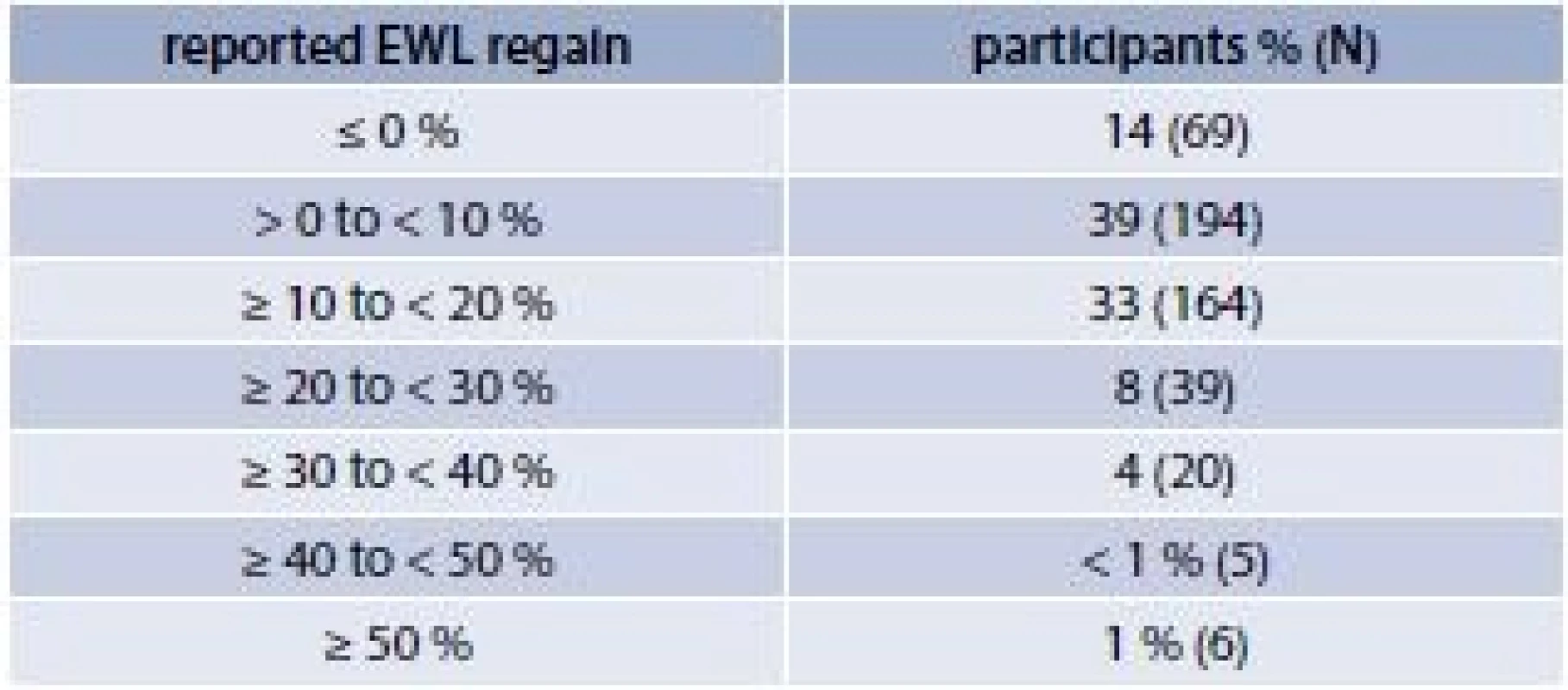
Bariatric surgery is currently the most effective treatment for severe obesity resulting initially in significant weight loss. Between the first and second years following surgery, weight loss often stabilizes and a substantial proportion of individuals begin to regain lost weight. In a study where 497 subjects who had undergone open or laparoscopic Roux-en-Y GBP surgery were followed, eighty six percent of the patients regained weight during an average follow-up time of 4.2 years (tab. 2) [3].
There is increasing evidence that patients who have problems with binge eating (BE) or BE disorder (BED) are quite common among the severely obese, including bariatric surgery candidates. A search was made of various databases regarding evidence of BE, BED and loss of control (LOC) eating post-operatively in bariatric surgery patients. Fourteen of the available 15 studies suggest that the development of problems with BE, BED or LOC eating post-bariatric surgery is associated with less weight loss and/or more weight regain post-bariatric surgery [4].
DNA sequence variations in eleven obesity candidate genes were tested to display the association with weight loss and weight regain in the Swedish Obese Subjects bariatric surgery cases. A total of 1443 subjects were analyzed for single-nucleotide polymorphisms (SNPs) from the following 11 genes: ADIPOQ (Adiponectin, C1Q and collagen domain containing), BDNF (Brain-derived Neurotrophic Factor), FTO (Fat mass and Obesity) , GNB3 (Guanine Nucleotide binding protein beta polypeptide), LEP (Leptin), LEPR (Leptin Receptor), MC4R (Melanocortin 4 Receptor), NR3C1 (Nuclear receptor subfamily 3, group C, member 1), PPARG (Peroxisome Proliferator Activated Receptor gamma), PPARGC1A (Peroxisome Proliferator Activated Receptor gamma coactivator 1 alpha) and TNF (Tumor Necrosis Factor alpha). General linear models were used to analyze associations between the SNPs and maximum weight loss and weight regain. The average maximum weight loss was 33.7 kg which was reached 2.2 years after the surgery. Subjects regained approximately 12 kg by year 6. After correcting for multiple testing, the FTO SNP rs16945088 remained significantly associated with maximum weight loss, as minor allele carriers lost approximately 3 kg less compared with common allele homozygotes. This association was particularly evident in the banding surgery patients, whereas no significant association was found in the gastric bypass subjects. No other SNPs were associated with maximum weight loss. There was no evidence that obesity-risk SNPs in FTO or other obesity candidate genes derived from genome-wide association studies are associated with maximum weight loss or weight regain over 6 years of follow-up in bariatric surgery patients [5].
Another study evaluated the influence of patient characteristics, preoperative weight loss, and type of surgical procedure on long-term weight loss after BS. Subjects were a prospective cohort of 95 patients who underwent BS with 4 years of follow-up. Seventy-seven patients (81.1 %) underwent laparoscopic Roux-en-Y gastric bypass, and 18 (18.9 %) had laparoscopic sleeve gastrectomy. Age, gender, initial BMI, preoperative percentage of excess weight loss, presence of type 2 diabetes, current smoking status, and surgical technique were analyzed via multivariate linear regression analysis to identify predictors of weight loss during the 4 years after the surgery. Maximum percentage of excess weight loss was obtained at 18 months. Age and preoperative BMI were negatively associated with percentage of excess weight loss at 1, 2, 3, and 4 years after BS. At 4 years, laparoscopic Roux-en-Y gastric bypass was independently associated with a higher weight loss than laparoscopic sleeve gastrectomy. Younger age, lower BMI, and laparoscopic Roux-en-Y gastric bypass were independent predictors of long-term weight loss after BS [6].
Roux-en-Y gastric bypass (RYGB) is a highly effective treatment for obesity and results in long-term weight loss and resolution of co-morbidities. However, weight regain may occur as soon as 1–2 years after surgery. A retrospective study aimed to investigate the prevalence of weight regain and possible preoperative predictors of this phenomenon after RYGB. A total of 1426 obese patients who had at least a 2-year follow-up were reviewed. Patients who were initially successful, having achieved at least 50 % excess weight loss at 1 year postoperatively were included. Patients were categorized into either the weight regain (WR) group or sustained weight loss (SWL) group based upon whether they gained more than 15 % of their 1-year postoperative weight. Weight regain was observed in 244 patients (17.1 %). Body mass index was significantly higher and percent excess weight loss was significantly lower in the WR group. Average weight regain was 19.5 kg and 8 kg in the WR and SWL groups, respectively. Time elapsed since RYGB was significantly longer in the WR group. Patients in the WR group were significantly younger, had fewer co-morbidities, and were less likely to have type 2 diabetes with insulin usage preoperatively. Unexpectedly, multiple co-morbidities and insulin-treated type 2 diabetes were factors associated with sustained weight loss in the study. Nevertheless, multivariate analysis could not identify these variables as independent predictors. This study confirmed that a longer interval after RYGB was associated with weight regain. Younger age was a significant predictor of weight regain even after adjusting for time since RYGB [7].
One study aimed to evaluate the relationship of well-documented (e.g., health, diet, physical activity) and theoretically relevant variables (e.g., substance use and “food addiction”) with both weight nadir and weight regain following BS. A sample of 97 Roux-en-Y gastric bypass patients (time since surgery 8.86 years) were surveyed about pre - and post-BS weight, health, self-management behaviors, alcohol problems, and clinical symptoms. Patients lost a mean of 42 % of total weight at weight nadir, but 26 % of the lost weight was regained by the time of the survey. Correlates of lower weight nadir and WR differed considerably, with minor overlap. Weight nadir was associated with pre-BS drug use and post-BS medical comorbidities. Weight regain was associated with post-BS adherence to dietary and physical activity modification. Post-BS nocturnal eating, depression, and problematic alcohol use were also associated with WR. With all associated variables in regression models, number of post-BS medical comorbidities and post-BS depression accounted for the most variance and remained as significant predictors of weight nadir and WR, respectively [8].
Data collected over 4 years from consecutive patients referred to a tertiary care bariatric center for upper endoscopy after RYGB was evaluated. Linear regression analysis was used to determine the association between the gastro-jejunal stoma diameter and weight regain. Among 165 patients 59 % had significant weight regain (≥ 20 % of maximum weight lost after the RYGB) and 41 % did not. The mean percent of maximal weight lost after RYGB that was regained in the entire cohort was 30 %. Gastro-jejunal stoma diameter was significantly associated with weight regain after RYGB surgery in univariate analysis. This association remained significant after adjusting for several risk factors for weight regain. Gastro-jejunal stoma diameter is a risk factor for weight regain after RYGB and can be incorporated in a novel prediction rule [9].
Numerous laboratory studies involving both animal and human models indicate that weight loss induces changes in leptin, ghrelin and insulin sensitivity, which work to promote weight regain. It is unclear, however, whether these biological changes serve as a biomarker for predicting weight regain in humans in which biological, behavioral and environmental factors are likely at play. Twelve studies reported changes in leptin, ghrelin or insulin during intentional weight loss with a follow-up period to assess regain. Two of the nine studies examining leptin suggested that larger decreases were associated with greater regain, three studies found the opposite, whereas four studies found no significant relationship. None of the studies supported the hypothesis that increases in ghrelin during weight loss were associated with weight regain. One study suggested that improvements in insulin resistance were associated with weight gain, but five subsequent studies reported no association. Changes in leptin, ghrelin or insulin sensitivity, taken alone, are not sufficient to predict weight regain following weight loss in humans [10].
Obese patients who underwent RYGB were studied for dietary habits by using 24 hour dietary recall and the Food Frequency Questionnaire. Rates of weight regain and the percentage of excess weight loss (EWL) were calculated. Patients were also asked whether they attended nutritional follow-up visits after the operation and about the type and regularity of physical activities. Weight regain was seen in 56 % of the patients with 29 % of the patients having regained over 10.1 % of the minimum weight reached after RYGB. Weight regain increased significantly with time after surgery. Poor diet quality characterized by excessive intake of calories, snacks, sweets, and fatty foods was statistically higher among those who regained weight. Sedentary lifestyle and lack of nutritional counseling follow-up were also significantly associated with regaining weight. Despite satisfactory results of EWL, the patients did not properly maintain the lost weight, mainly after 5 years postsurgery [11].
Conclusion
It is important to identify individuals with high chances for weight loss and who are at high risk for weight regain. Whatever predictors have been proposed so far for weight loss, maintenance and weight regain, patients’ adherence to treatment and multidisciplinary post-operative follow-up programs remains to be essential. Future prospective studies are needed to further explore the prevalence, predictors, and mechanisms of weight loss and weight regain after bariatric surgery.
prof. Dr. Volkan Demirhan Yumuk
http://cerrahpasa.istanbul.edu.tr/en/
Received: 2. 3. 2018
Accepted: 5. 4. 2018
Sources
- Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 2017; 376(3): 254–266. Available on DOI: <http://dx.doi.org/10.1056/NEJMra1514009>.
- Karmali S, Brar B, Shi X et al. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg 2013; 23(11): 1922–1933. Available on DOI: <http://dx.doi.org/10.1007/s11695–013–1070–4>.
- Kofman MD, Lent MR, Swencionis C.. Maladaptive Eating Patterns, Quality of Life, and Weight Outcomes Following Gastric Bypass: Results of an Internet Survey. Obesity (Silver Spring) 2010; 18(10): 1938–1943. Available on DOI: <http://dx.doi.org/10.1038/oby.2010.27>.
- Meany G, Conceição E, Mitchell JE. Binge Eating, Binge Eating Disorder and Loss of Control Eating: Effects on Weight Outcomes after Bariatric Surgery. Eur Eat Disord Rev 2014; 22(2): 87–91. Available on DOI: <http://dx.doi.org/10.1002/erv.2273>.
- Sarzynski MA, Jacobson P, Rankinen T et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond) 2011; 35(5): 676–683. Available on DOI: <http://dx.doi.org/10.1038/ijo.2010.166>. Erratum in Int J Obes (Lond). 2012; 36(7): 1016.
- Parri A, Benaiges D, Schröder H et al. Preoperative predictors of weight loss at 4 years following bariatric surgery. Nutr Clin Pract 2015; 30(3): 420–424. Available on DOI: <http://dx.doi.org/10.1177/0884533614568154>.
- Shantavasinkul PC, Omotosho P, Corsino L et al. Predictors of weight regain in patients who underwent Roux-en-Y gastricbypass surgery. Surg Obes Relat Dis 2016; 12(9): 1640–1645. Available on DOI: <http://dx.doi.org/10.1016/j.soard.2016.08.028>.
- Yanos BR, Saules KK, Schuh LM. Predictors of Lowest Weight and Long-Term Weight Regain Among Roux-en-Y Gastric Bypass Patients. Obes Surg 2015; 25(8): 1364–1370. Available on DOI: <http://dx.doi.org/10.1007/s11695–014–1536-z.>.
- Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal Stoma Diameter Predicts Weight Regain after Roux-en-Y Gastric Bypass. Clin Gastroenterol Hepatol 2011; 9(3): 228–233. Available on DOI: <http://dx.doi.org/10.1016/j.cgh.2010.11.004>.
- Strohacker K, McCaffery JM, MacLean PS et al. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes 2014 ; 38(3): 388–396. Available on DOI: <http://dx.doi.org/10.1038/ijo.2013.118>.
- Freire RH, Borges MC, Alvarez-Leite JI et al. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012; 28(1): 53–58. Available on DOI: <http://dx.doi.org/10.1016/j.nut.2011.01.011>.
Labels
Diabetology Endocrinology Internal medicineArticle was published in
Forum Diabetologicum

2018 Issue 2
-
All articles in this issue
- The current situation in the management of obese patients in Slovakia: the concept of national comprehensive obesity management in the Slovak Republic
- The management of the obesity-induced hypertension
- Is obesity a real cardiovascular risk factor?
- Obesity paradox in patients with heart failure
- Nonalcoholic fatty liver disease and metabolic syndrome
- Can bariatric surgery help in the treatment of obese type 2 diabetic patients?
- Predictors of weight loss and weight regain after bariatric surgery
- Management after bariatric surgery
- Forum Diabetologicum
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Nonalcoholic fatty liver disease and metabolic syndrome
- The current situation in the management of obese patients in Slovakia: the concept of national comprehensive obesity management in the Slovak Republic
- Can bariatric surgery help in the treatment of obese type 2 diabetic patients?
- Obesity paradox in patients with heart failure
